Demystifying Neon 20: Understanding Its Mass Number
How To Find The Mass Of One Atom Of Neon (Ne)
Keywords searched by users: What is the mass number for Neon 20 neon-20 neutrons, neon 20 atomic mass, neon 20 isotope, neon-20 symbol, average atomic mass of neon-20 21 22, what is the mass number of neon, neon 21, relative atomic mass of neon 21
What Is The Mass Of Neon 20?
Neon, a chemical element, is composed of three naturally occurring isotopes, each with a distinct mass. One of these isotopes is Ne-20, which constitutes the majority of neon found in nature, making up approximately 90.92% of a typical neon sample. Ne-20 is characterized by having 10 neutrons in its nucleus and a mass of approximately 19.99 atomic mass units (amu). This information helps us understand the specific composition of neon and its most abundant isotope, Ne-20, providing insight into the element’s atomic structure.
What Is The Mass Number Of Neon 20 Or 21?
What is the mass number of neon isotopes, specifically neon-20 and neon-21? The mass number of an isotope represents the total number of protons and neutrons in its nucleus. Neon, a chemical element with the atomic number 10, has several isotopes with varying mass numbers. Neon-20 has a mass number of 20, meaning it has 10 protons and 10 neutrons in its nucleus. Neon-21, on the other hand, has a mass number of 21, indicating it has 10 protons and 11 neutrons in its nucleus. These isotopes of neon differ in their neutron count, which affects their atomic mass and stability.
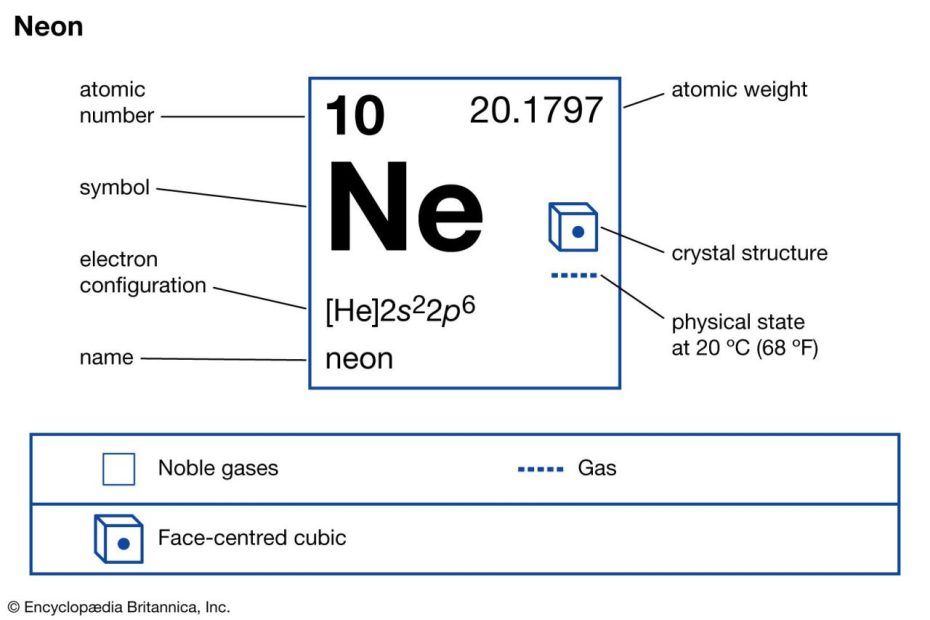
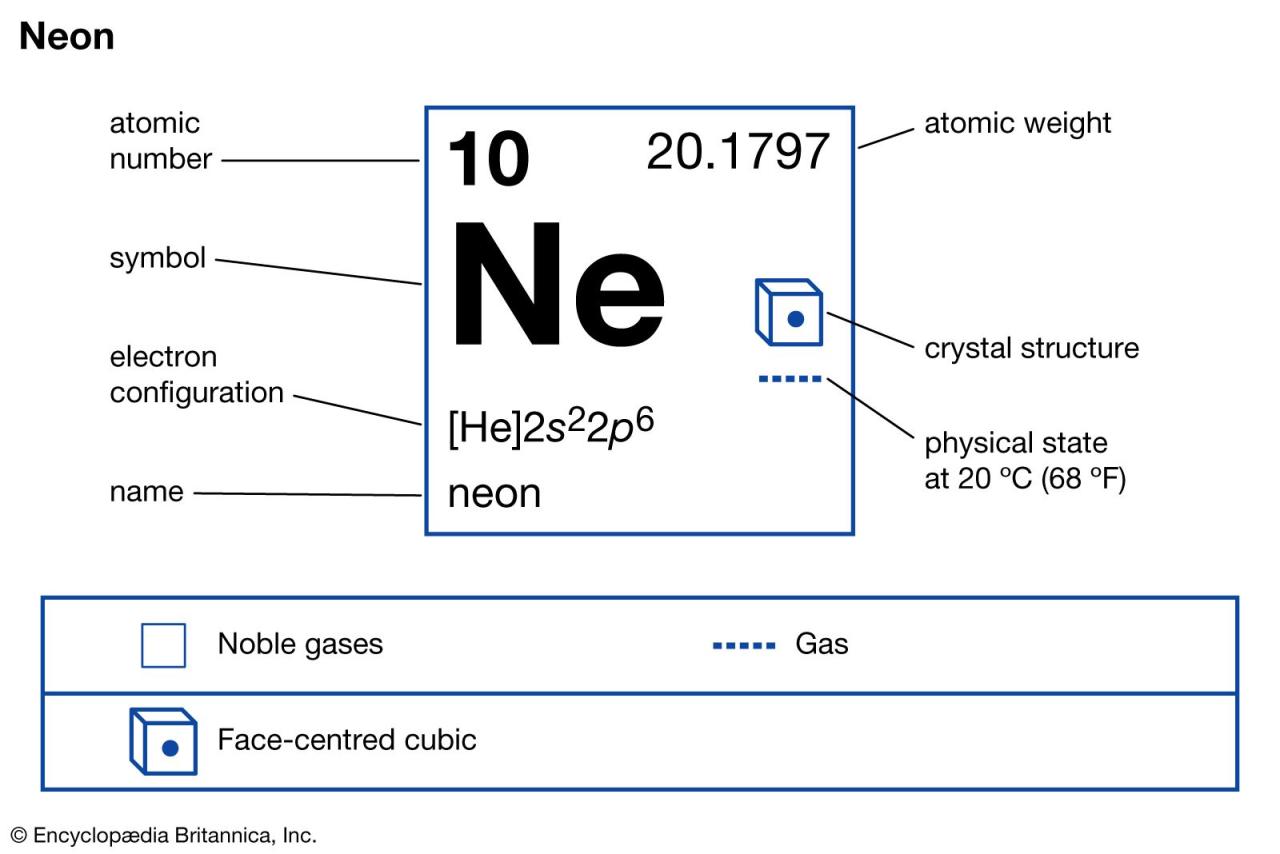
What Is The Atomic Number For Neon 20?
The atomic number for neon is 10. Neon, represented by the symbol Ne, belongs to Group 18 of the periodic table. It has a melting point of -248.59°C, which is equivalent to -415.46°F, or 24.56 K. Neon is classified as a noble gas and is found in the p-block of the periodic table. Its atomic mass is approximately 20.180 atomic mass units. The electron configuration of neon is [He] 2s^2 2p^6.
Top 41 What is the mass number for Neon 20





Categories: Discover 87 What Is The Mass Number For Neon 20
See more here: trainghiemtienich.com

2: Neon Isotopes. Neon has three naturally occurring isotopes. In a sample of neon, 90.92% of the atoms are Ne-20, which is an isotope of neon with 10 neutrons and a mass of 19.99amu.Hence, the atomic mass of Neon is 20.18 amu.
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 20Ne | 19.992 440 18(1) | 0.9048(3) |
| 21Ne | 20.993 8467(3) | 0.0027(1) |
| 22Ne | 21.991 3851(1) | 0.0925(3) |
| Group | 18 | −248.59°C, −415.46°F, 24.56 K |
|---|---|---|
| Block | p | 0.000825 |
| Atomic number | 10 | 20.180 |
| State at 20°C | Gas | 20Ne |
| Electron configuration | [He] 2s22p6 | 7440-01-9 |
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
|---|---|---|
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
| 21 | Scandium | 44.956 |
Learn more about the topic What is the mass number for Neon 20.
- Atomic Weight of Neon | Commission on Isotopic … – ciaaw
- 4.9: Atomic Mass: The Average Mass of an Element’s Atoms
- What is the atomic mass of Neon?
- Neon – Element information, properties and uses | Periodic Table
- Atomic Mass of First 30 Elements – BYJU’S
- [Solved] A neon20 atom has a mass of 332 1026 kg a What temperature in K