Exploring The Fundamental Differences: What Sets Pure Substances Apart From Mixtures
Types Of Matter – Elements, Compounds, Mixtures, And Pure Substances
Keywords searched by users: What makes pure substances different from mixture what makes pure substance different from mixture brainly, what makes a substance a mixture?, what makes a mixture different from other types of matter, difference between pure substance and mixture examples, what trophic level does the plant belong to, modern medicine is one of the outstanding, 5 examples of pure substances found at home, what is the difference between substance and pure substance
How Is A Pure Substance Different From A Mixture Brainly?
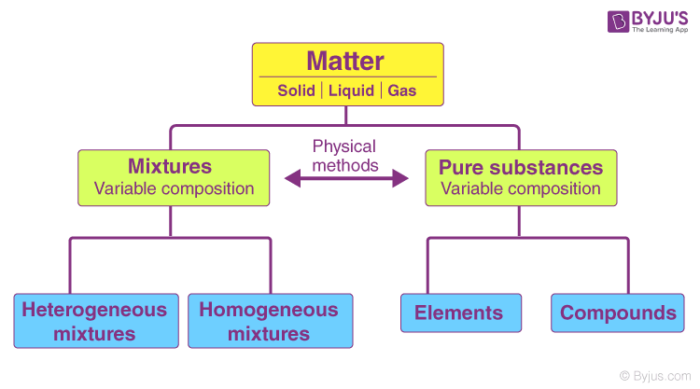
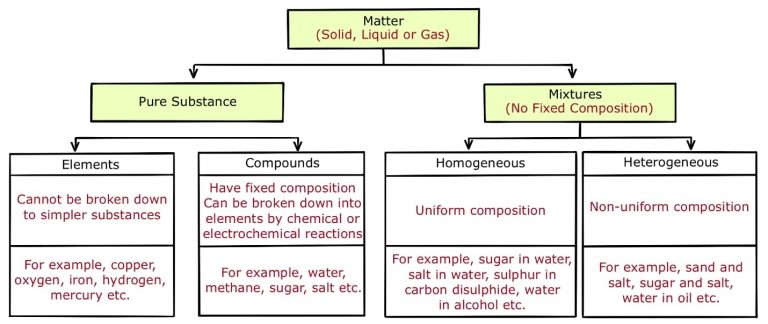
A pure substance is fundamentally distinct from a mixture due to its composition. Pure substances are either compounds or elements. Compounds are composed of different atoms chemically bonded together, while elements consist of identical atoms. In contrast, mixtures comprise a combination of various substances that are physically blended together, without undergoing chemical bonding. This distinction is crucial in understanding the fundamental nature of matter. This information was provided on October 26, 2020.
What Is The Difference Between A Pure Substance And A Mixture Quizlet?
Let’s clarify the distinction between pure substances and mixtures. A pure substance consists of particles that are all the same type. For instance, table sugar is considered a pure substance because it contains only sugar particles, and distilled water is also a pure substance because it consists solely of water particles. On the other hand, mixtures are combinations of pure substances. In a mixture, you’ll find at least two different types of particles. To illustrate, a salad is a mixture because it contains various ingredients like lettuce, tomatoes, and cucumbers, each made up of distinct particles. In summary, the key difference lies in the composition: pure substances have uniform particles, while mixtures comprise multiple types of particles coming together.
What Is The Difference Between Pure And Pure Substance?
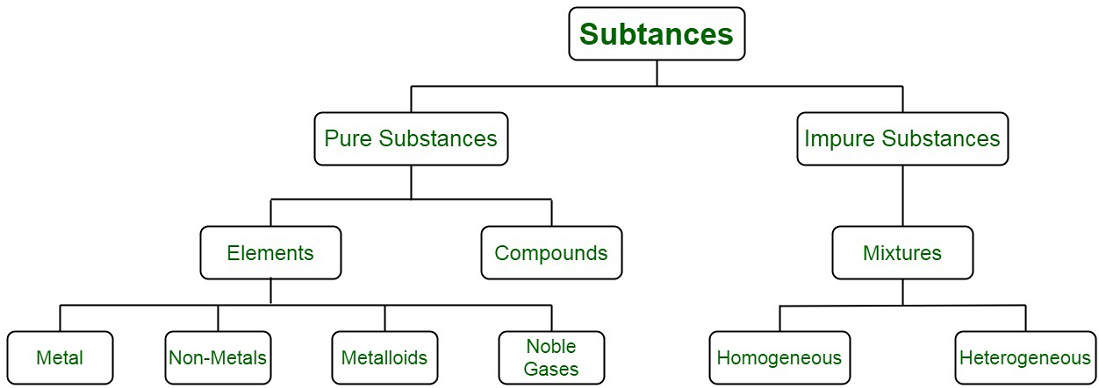
To grasp the distinction between pure substances and impure substances, it’s essential to consider their fundamental composition. Pure substances consist solely of one specific type of atom or molecule, maintaining a uniform chemical structure throughout. In contrast, impure substances, also known as mixtures, consist of various types of atoms or molecules intermingled together. Impure substances can be further categorized into two primary groups: elements and compounds.
Elements, within the realm of impure substances, represent the most basic constituents of matter. Each element consists of a single type of atom, such as oxygen (O) or hydrogen (H), and cannot be further broken down into simpler substances through chemical means.
Compounds, on the other hand, are also impure substances but are formed when different types of atoms chemically combine in fixed ratios. These combinations result in distinct molecules with unique properties, such as water (H2O) or carbon dioxide (CO2). In compounds, the constituent atoms are bonded together in specific arrangements, giving rise to diverse chemical properties compared to their individual elements.
So, in summary, the primary difference between pure substances and impure substances lies in their composition: pure substances consist of a single type of atom or molecule, while impure substances encompass a variety of atoms or molecules, which can further be classified as elements or compounds. Understanding this distinction is fundamental to grasping the nature of matter and its chemical behavior.
Details 17 What makes pure substances different from mixture



Categories: Discover 50 What Makes Pure Substances Different From Mixture
See more here: trainghiemtienich.com

A pure substance is a single kind of matter that cannot be separated into other kinds of matter by any physical means. A pure substance always has a definite and constant composition. A mixture is a physical combination of two or more pure substances in which each substance retains its own chemical identity.Answer: pure substances are compounds and elements (made up of the same atom or same molecule respectively), while mixtures are an assortment of different substances put together.A pure substance is made up of only one kind of particle. For example, table sugar only contains sugar particles and distilled water contains only water particles. Mixtures are made up of pure substances combined together. A mixture is composed of at least two different kinds of particles.
Learn more about the topic What makes pure substances different from mixture.
- What is Pure Substance? – Definition, Examples, Difference …
- what are the difference between pure substance and Mixture? – Brainly.ph
- Pure Substances and Mixtures Part 2 Flashcards | Quizlet
- Difference between pure and impure substances and their properties
- How does a homogeneous mixture differ from a pure substance … – Vaia
- What is the difference between mixtures and substances? – Quora